Evacuations from Israel and High-Risk Locations Call +44 (0)1202 308810 or Contact Us →
COVID-19: The Fight Against the Pandemic
2 Jun 2020
With the number of COVID-19 cases globally exceeding 6 million and billions of people living under lockdowns, the attention of many governments has turned to the possibility of easing restrictive social-distancing measures and restarting economic activity. This has brought about the need for different ways to fight the virus. Whilst mass testing and sophisticated contact tracing infrastructure will aid in the suppression of COVID-19 in the coming months, life will not return to the pre-pandemic normal until effective treatments and vaccines are developed.
Key Points
• Having effectively suppressed the initial wave of the virus through strict social distancing measures, many nations are moving towards gradually easing lockdown measures. However, with the virus still posing a considerable risk, the re-imposition of restrictions cannot be ruled out in any country.
• There has been a significant increase in testing capacity worldwide, with governments working to develop contact tracing infrastructure in order to prevent unmanageable second waves of infection.
• A number of treatments for COVID-19 are in development, with some existing drugs being repurposed. It is likely that an effective treatment will involve both the use of drugs and measures to prompt an immune response in the body.
• With hundreds of candidate vaccines in development, it is possible that an effective vaccine for COVID-19 will be discovered by the autumn of 2020. There is, however, a realistic probability that a vaccine will not be available for another 12-18 months, if at all.
Easing Lockdowns
As nations consider the ongoing economic and social damage that has been caused by lockdowns, and the increasing cost of measures designed to prevent mass unemployment, many have begun to ease the strictest social distancing measures in order to allow some form of economic activity to resume. Key to these considerations is that economic malaise in itself can trigger a general decline in public health and therefore increase excess deaths. This effect likely being pronounced in less developed nations where a large proportion of workers are employed in the informal economy.
Additionally, there will also be a cost in lives resulting from the rebalancing of healthcare systems away from routine treatments in favour of treating COVID-19 patients, as well as the reluctance of people suffering the symptoms of other serious conditions to present themselves at medical facilities.
Governments seeking to ease social distancing measures, which have been extremely successful in slowing transmission of the virus, are generally striving to achieve a balance between suppressing the virus and allowing economic activity to resume in order to both keep COVID-19 deaths to a minimum and avoid excess deaths due to poor public health and economic decline in the coming years.
It is worth noting that while lockdown measures across the world were introduced as a matter of scientific imperative, with little opposition, the way countries emerge from their individual lockdowns has been more political. Polling in a number of countries has indicated that the previously observed ‘rally round the flag’ effect, where governments saw high approval ratings, has now begun to recede as administrations revert to their political and economic ideologies to chart a course out of lockdowns, as a sense of “fatigue” sets in among the general public who are seeing much of their liberties curtailed. This in turn has brought about the return of traditional oppositional politics.
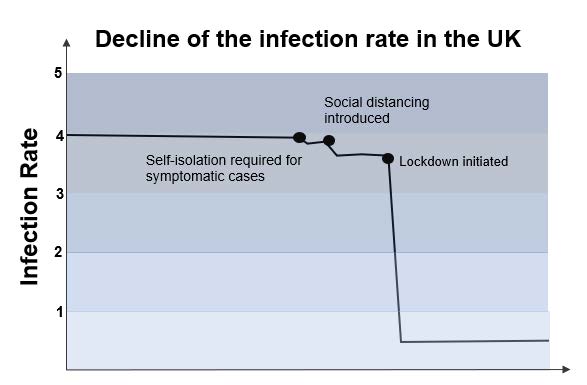
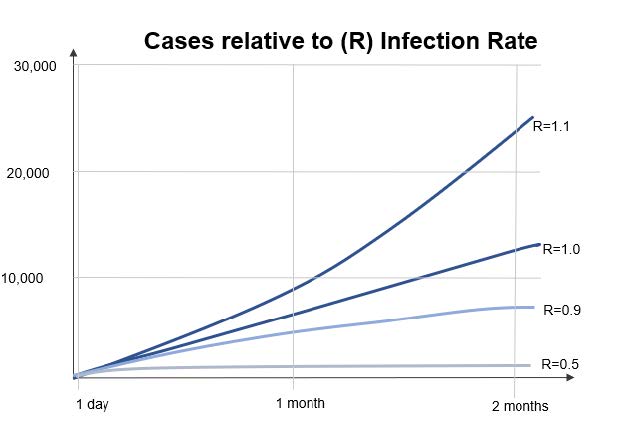
The continued decisions on how to ease lockdown measures in the near term will likely be based on the basic reproduction number, commonly known as the R number or R nought (R0). This is an epidemiological term referring to the expected number of cases directly generated by one case in a population where all individuals are susceptible to infection. If the R number is above 1 then each infected individual is expected to infect, on average, more than one other person, therefore ensuring the continued growth in the number of active cases within the population.
It is believed that, without significant intervention, the R number of COVID-19 lies between 2.5 and 4, meaning that each case generates between 2.5 and 4 new cases. Government interventions in many countries in recent months have succeeded bringing the R number below 1, meaning that each case, on average, generates less than one new case, thus decreasing the overall number of active cases and controlling the spread of the outbreak. As such, governments are currently considering which restrictions can be eased without pushing the R number above 1.
The importance of consistently keeping R below 1 to avoid a damaging resurgence of the virus cannot be overstated. Indeed, German Chancellor Angela Merkel has indicated that a sustained R number of just 1.1 would result in there being more patients requiring intensive care than available beds in around 4-5 months in the country.
Officials will be aware of the experience of the Japanese island prefecture of Hokkaido, which, after successfully suppressing the virus, removed almost all restrictions and subsequently saw a resurgence in the virus that was more severe that the initial peak. Hokkaido was then forced to reimpose a second lockdown after just three weeks. Many governments are keen to avoid having to impose such actions on their countries.
This requires an amount of flexibility from governments. For example, South Korea, a relative success story of the outbreak has not been without its own setbacks. The government has combined an aggressive testing regime, a robust track and trace system and a slow, multi-phased winding down of restrictions to reopen its economy. This approach has shown some initial success; however, some restrictions had to be re-imposed following a number of new cases linked to Seoul’s nightlife scene. Additionally, more recently, hundreds of schools have also had to close just days after reopening following a spike in cases. Despite this, and more importantly, this demonstrates the government as responsive as it remains guarded against a second wave of the virus and has measures in place, such as mandatory 14-day quarantines for those entering the country, in an effort to prevent a large-scale re-emergence of the virus.
The majority of world leaders appear to favour a gradual easing of lockdown measures, similar to South Korea, with perceived lower risk actions, such as allowing younger children to attend school a first step. A slow, multi-stage easing of lockdown measures based on both the R number and the calculated prevalence of the virus in the general public does also, crucially, have the support of much of the scientific community when combined with a high-capacity test and trace system. The support of the scientific community is seen as vital for securing the confidence of a nervous general public, who may be reluctant to return to work if they perceive their personal health and safety to be at risk. Additionally, many economists have opined that having to reimpose harsh lockdown restrictions following a period of easing would likely possibly trigger a loss in business confidence, investor flight and significant economic damage.
In short, nations that take a cautious approach to reopening their economies following a sustained suppression of the virus are more likely to prevent a resurgence in the virus and therefore fare better economically in the short to medium term. It is, however, also likely that nations will see a rise in COVID-19 cases as restrictions are eased. Governments are for the most part looking to the development of mass testing and contact tracing infrastructures in order to suppress localised infection clusters and therefore avoid the reposition of blanket lockdown measures.
Testing
From the beginning of the COVID-19 crisis, the World Health Organisation (WHO) indicated that an aggressive mass testing regime coupled with a robust contact tracing infrastructure is key to fighting the virus and will be imperative in countries that are loosening lockdown measures. This is borne out by the experiences of a number of countries that entered the crisis with existing mass diagnostic testing infrastructure in place. Many nations that initially struggled on this front have built large testing infrastructures rapidly. New testing methods have been developed to circumvent the global shortage of materials needed for the traditional Polymerase Chain Reaction (PCR) method. Large scale testing paired with app-based contact tracing is being employed in a number of advanced nations with some success.
Key to the initial success of both Germany and South Korea in containing the first wave of COVID-19 has been an in-place large diagnostics industry. South Korea was particularly well-prepared for the arrival of COVID-19, having learned lessons from a previous outbreak of Middle East Respiratory Syndrome (MERS) in 2015. Other countries, such as the United Kingdom, struggled in the early stages of the pandemic to carry out large amounts of tests due to a small indigenous diagnostic infrastructure.

The ability of countries to increase testing capacity was also hampered by a shortage of the chemical reagent necessary to identify the virus when using PCR testing. However, the rapid development of new methods of testing has allowed many to dramatically increase their capacity to identify individuals infected with COVID-19. For example, both the UK and Russia have seen a massive increase in their testing capacity since their outbreak began.
In addition to tests to determine whether an individual is currently infected with COVID-19, much emphasis has been placed on antibody testing to determine whether individuals have had and recovered from the virus. These tests are seen as highly consequential as it is likely that an individual who has recovered from the virus has, in theory, a measure of immunity from further infection. Currently, lab-based tests are available to detect the presence of antibodies in a blood sample. However, it is likely that governments will face considerable logistical challenges when trying to scale up lab-based antibody testing. An antibody test developed by Swiss firm Roche has received clinical approval from regulators in the US, EU and UK, with a reported 99.8 per cent accuracy. Though lab-based, the Roche antibody test can deliver results in as little as 18 minutes, meaning it may have the capability of being delivered at a larger scale than currently available.
Dozens of biotech firms are currently marketing at-home antibody testing kits, in which governments have shown considerable interest. In principle, these tests lend themselves to mass distribution by mail, with some governments placing provisional orders for millions of kits. However, no test has, as yet, been found to be sufficiently accurate for public distribution. In light of this, several governments are working with test manufactures to develop reliable and accurate home testing kits, with results likely to emerge in a matter of months rather than weeks.
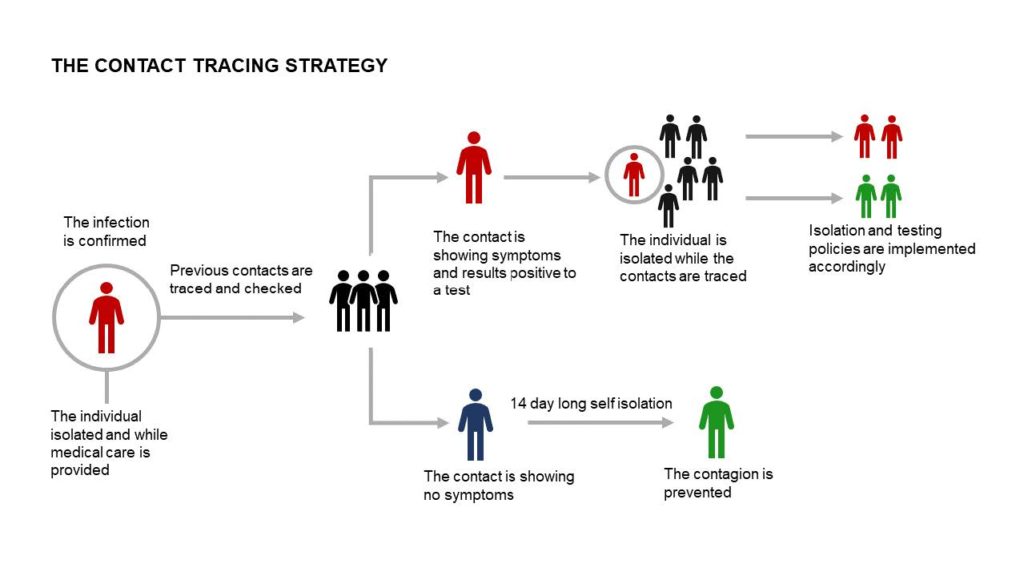
A large testing capacity in itself, however, is only a part of the infrastructure required to effectively tackle COVID-19. Many nations are looking to employ a system of ‘test, trace, isolate,’ where the contacts of a person found to be infected are traced, isolated and themselves tested in an effort to break the chain of infection. Whilst Western nations are in the most part looking to smartphone technology to trace the contacts of those involved, many other nations, such as South Africa, are relying on existing public health bodies who have gained experience in contact tracing following outbreaks of other diseases such as Ebola and HIV.
A number of governments have rolled out smartphone apps that use Bluetooth to determine who has been in contact with an infected individual. China has demonstrated that this technology can be used to effectively suppress the virus and break chains of transmission. However, uptake of these apps has been slow in more liberal societies, where concerns about data security have been raised by privacy activists. Singapore, for example, has seen just 20 per cent uptake of its contact tracing app. Researchers believe that uptake needs to be above 60 per cent for such technology to be at its most effective. A possible solution to low uptake is a joint venture between tech giants Google and Apple that installs tracing technology directly into the operating systems of all smartphones running both iOS and Android, without the user having to actively download an app.
Treatments
Slowing the spread of the virus is not the only step that governments need to take to combat the pandemic, effective treatment of COVID-19 is vital to lower the risk and allow for the further relaxation of measures and for the return of a semblance of pre-pandemic normality. To this end, a number of potential therapeutic treatments have emerged. Broadly, treatments being assessed take one of two approaches. While some existing drugs are being trialled to directly fight the virus, other potential treatments are focussed on encouraging the human body’s organic immune response. The WHO is currently coordinating a large worldwide clinical trial, known as the solidarity trial, aimed at exploring the possibility of repurposing existing drugs to treat COVID-19. As yet, very few treatments have received formal authorisation for use on COVID-19 patients.
Firstly, antiviral drug remdesivir has shown promise as a potential treatment for the virus, having received an emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) in April 2020 and UK clinical approval in May 2020. The EUA was granted after preliminary results of a medical trial revealed that the drug had a positive effect on the time patients take to recover from severe cases of COVID-19. The research, which has now been verified by peer review, shows that remdesivir shortened the course of the illness from an average of 15 days to about 11 days.
However, the drug did not appear to influence the overall mortality associated with COVID-19, meaning that it did not save the life of anyone who otherwise would have died. Current evidence suggests that, although useful, remdesivir alone is not a ‘silver bullet.’ The ability of remdesivir to shorten the course of COVID-19 will possibly prove useful in countries where health services are finding themselves struggling to cope with an influx of patients, as such helping the overall fight against the pandemic. Some in the medical research community opine that remdesivir may be useful alongside a treatment designed to boost the body’s immune response, such as blood plasma therapy.
Indeed, a small study in the US indicated that patients with severe COVID-19 and given plasma from someone who had recovered from the disease were more likely to stabilize or need less oxygen support than other similar hospital patients. The use of blood from people who have already recovered from a disease is a well-established approach and has seen some success in the past, notably in the fights against COVID-19’s sister diseases, SARS and MERS. A large-scale trial using convalescent plasma transfusions is currently underway, with results expected in the coming weeks. Whilst the treatment is yet to receive approval from health regulators, the US FDA has been assessing its efficacy and safety and is likely to reach a decision in the coming weeks.
Scientists in Germany are currently exploring a therapy using an enhanced tuberculosis vaccine that would boost general immunity to infectious respiratory diseases. The candidate vaccine, known as VPM1002, is not specifically targeted at COVID-19 and is unlikely to be a full vaccine, but it is thought that it reduces the severity of infections and the overall death rate attributable to the virus. Clinical trials involving thousands of volunteers, including a cohort of over-60s, are currently ongoing. Because of its similarity to the existing BCG tuberculosis vaccine, VPM1002 could be produced in large quantities within a short period of time and could be used to bridge the gap until such time as an effective COVID-19 vaccine is found.
However, there have been some high-profile failures in the research into the treatment of COVID-19. Touted as a ‘game-changer’ by US President Donald Trump, who even said he was taking it, antimalarial drug hydroxychloroquine has had its trial paused by the WHO. The suspension of the trial comes after a new study found a significantly higher risk of death among those taking hydroxychloroquine or chloroquine, which is closely related. It is well documented that the drugs can cause irregular heart rhythms. It appears that Trump latched on to the potential treatment following a small French study, that has been subsequently withdrawn, that suggested that the drugs significantly reduced death rates in hospitalised COVID-19 patients.
Vaccine
Even as COVID-19 is brought under control in many countries and potential treatments are found, the virus remains a serious threat until a degree of ‘herd immunity’ is achieved. Whilst this can be achieved by allowing the virus to infect large amounts of people, this approach would likely lead to the deaths of millions of vulnerable and immunocompromised individuals across the world. Modelling presented to the UK government suggested that this approach could lead to 500,000 deaths in UK alone.
Herd immunity can also be obtained through widespread vaccination. In the past, diseases such as smallpox and polio have been practically eradicated by mass vaccination. There are currently hundreds of potential vaccines in development, with candidate vaccines in the UK, Europe, the US and China showing particular promise. The process of developing vaccines, however, is long and laborious, often taking years or decades. The fastest vaccine ever developed was for mumps, taking around four years. It is also worth noting that there is no guarantee that a suitable vaccine will be found. Indeed, more than 30 years after HIV was identified and isolated, scientists have thus far failed to find a suitable vaccine to protect against infection.
A small-scale trial of a candidate vaccine in Wuhan, the Chinese city where COVID-19 originated, has rendered positive results. The vaccine, which was trialled on around 100 people, generated varying levels of immune response in the volunteers, with most developing a type of antibody that can attach to the virus, though not necessarily destroy it. Some also developed so-called neutralising antibodies, which can kill the virus. The candidate vaccine, which has been developed by CanSino Biologics, is due to enter large scale trials in in Canada in the near future.
American biotech firm Moderna has announced that it has developed a safe candidate vaccine that stimulated an immune response in a small sample of volunteers. Importantly, two doses of the vaccine prompted the production of a similar level of neutralising antibodies as in people who have recovered from COVID-19. Researchers involved in the development of the Moderna vaccine have indicated that it is conceivable that the candidate may be available to the general public by December 2020.
In the UK, the government has invested £84 million (US$ 104 million) in vaccine development projects at Oxford University and Imperial College London. Researchers developing the Oxford candidate vaccine have been quoted as being around 80 percent sure that their candidate, known as Chaddox, is both safe for use and effective at preventing COVID-19 infection. Indeed, the vaccine stimulated antibody production when given to rhesus macaque monkeys. Thousands of people are currently taking part in the Chaddox human trial, with at least 10,000 due to receive test inoculations in the near future. Researchers, however, have expressed concern that diminishing levels of COVID-19 in the UK mean that it may be difficult to determine whether Chaddox is an effective inoculation against the virus. The UK pharmaceutical firm AstraZenaeca has partnered with the Oxford Vaccine Group to manufacture the vaccine in large numbers should the trials prove successful, with plans in place to deliver 100 million doses by September 2020. Results from large scale trials of Chaddox are likely to be known in the coming weeks.
With over 7.5 billion people on the planet, most of whom will require vaccination. General scientific consensus is that a number of candidate vaccines are required to be successful in the coming months for the world to avoid a prolonged exit from the current crisis.
Conclusion
As governments begin easing the restrictive social-distancing measures and restarting their economies, emphasis is now being put on fighting the virus and getting life back to some semblance of normality. The success of social distancing, testing and treatment are all vital to returning life to a semblance of normality, as well as avoiding severe economic hardship.
However, until an effective vaccine is found, or the pandemic has run its course and achieved herd immunity at great economic and human cost, societies may have to accept a “new normal” in the way of living, working and interacting.